Electronegativity
& Polar Covalent Bonds:
Some
elements tend to attract electrons more strongly than others. This
property is roughly described as "electronegativity." If two atoms
of differing electronegativity form a bond, the electrons spend more time
on the more electronegative atom. In the extreme, we have an ionic
bond.
However,
it should be noted that no bond can be 100% ionic--there is always some
sharing of electrons between atoms. For instance, NaCl is about 80%
ionic and 20% covalent. Conversely, polar covalent bonds can be thought
of as paritially ionic (such as the bond in HCl which is often mentioned
to be about 10% ionic and 90% covalent).
Ionic
and covalent are useful concepts but it must be remembered that they are
extremes and that many bonds lie somewhere in between. Only a nonpolar
covalent bond is close to 100% pure (and, even then, some valence bond
structures of these can be partially ionic).
Problem
7.38: What general trend in electronegativity occur in the
periodic table?
What
we shall do here is first show another modified periodic table. This
time electronegativities are shown for all the elements. We have
both a number for each one and a direct indication of trends as a whole.
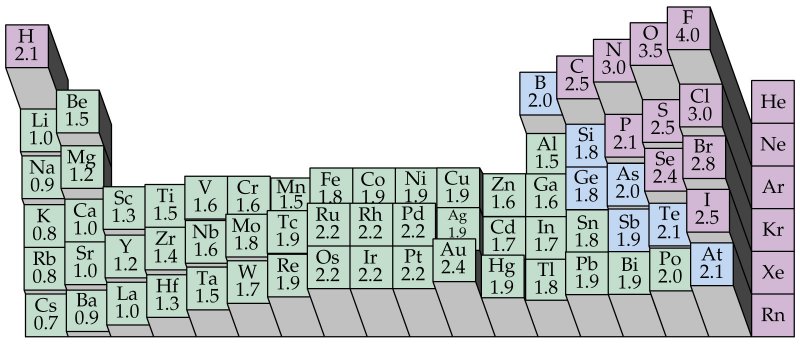
Obviously
the trends are now easily stated:
-
Electronegativity
increases from left to right across a period (excluding group 8A, the snobbish
gases).
-
Electronegativity
decreases down a group. But...
-
Be
careful here! Note that, with transition elements, it increases going
down for some groups.
-
What
happens with the 8B elements (Fe, Co, and Ni and their relatives below
them, Ru-Rh-Pd and Os-Ir-Pt) is particular interesting in that these are
all pretty close to each other; these three sets of elements are collectively
known as the triads and they have a great many things in
common.
-
The
1B elements, Cu, Ag, and Au, are also interesting in their behavior.
The high electronegativity of Au (gold) is of particular interest in some
applications.
-
By
the time we get to group 2B (Zn, Cd, Hg) things are beginning to "flatten
out."
-
Things
are back to normal and "pHull of pHun" when we go further right with the
group 3A elements and beyond.
-
Electronegativities
are in some respects heuristic (hand-waving) numbers.
But for quick estimates of things, they are very very useful!
Problem
7.39: Predict the electronegativity of the undiscovered element
with Z = 119.
Again,
we show the chart. This has two purposes:
-
It
makes things better for those of you who like to use the web pages.
-
It
makes things more difficult for those who insist on printing everything
out on paper. (I really like getting the collective goat of those
people!)
Here
is the chart again: We note that element #119 would be directly below
francium.

All
we need do is look at the left-most column. We see the following
numbers:
|
Li
|
1.0
|
|
Na
|
0.9
|
|
K
|
0.8
|
|
Rb
|
0.8
|
|
Cs
|
0.7
|
Just
extrapolating, and noting that the increase in atomic number, if plotted
vs. this would look rather shallow (the atomic numbers go
as 3, 11, 19, 37, 55). Fr is 87 and we would probably predict its
electronegativity as 0.7 or 0.6. (Since we only go to +0.1
in the estimates, these are probably good numbers.)
The
end result here is that we would probably set the E.N. of element #119
to be about 0.6. This is quite low but one would expect it to be
the least electronegative of ALL elements in any case.
Problem
7.42: Which of the following substances are largely ionic
and which are covalent?
With
electronegativities, there is a fairly simple, albeit crude, rule:
Between
two atoms:
-
If
DEN
> 2, the bond is primarily ionic.
-
If
2 > DEN
> 0, the bond is polar covalent.
-
If
DEN
= 0, the bond is pure covalent (or close to it).
We
apply these rules to the bonds given. In order to facilitate things
for the web students and to aggravate those who insist on having hard copy
for everything, we again show the E. N. periodic table figure:

Now,
we cruise forth and handle the bonds. Just use the numbers above
to get those below.
| (a) |
HF |
ENF
= 4.0; ENH = 2.1:
DEN = 1.9 |
HF is polar covalent. |
| (b) |
HI |
ENI
= 2.5; ENH = 2.1:
DEN = 0.4 |
HI is polar covalent. |
| (c) |
PdCl2 |
ENCl
= 3.0; ENPd = 2.2:
DEN = 0.8 |
PdCl2
is polar covalent. |
| (d) |
BBr3 |
ENBr
= 2.8; ENB = 2.0:
DEN = 0.8 |
BBr3
is polar covalent. |
| (e) |
NaOH |
Na+-OH-
is ionic!
So, we look at the
hydroxide
ion:
ENO
= 3.5; ENH
= 2.1: DEN
= 1.4 |
OH-
is polar
covalent. |
| (f) |
CH3Li |
Here, we know that the C-H bond
is polar
covalent (with just a difference
of 0.4). So,
what about C-Li? We look
at this now.
ENC
= 2.5; ENLi = 1.0:
DEN = 1.5 |
CH3Li
is polar covalent. |
There are lots and lots
of bonds which are polar covalent. Just a few are ionic!
Problem
7.43: Use the electronegativity data in Figure 7.4 to predict
which bond in each of the following pairs is more polar.
Just
use the chart for these! In fact, here it is again (to help you and
to infuriate the folks who insist on printing out everything):

| (a) |
C-H or C-Cl |
Respective DEN's:
0.4 & 0.5 |
C-Cl is more polar |
| (b) |
Si-Li or Si-Cl |
Respective DEN's:
0.8 & 1.2 |
Si-Cl is more polar. |
| (c) |
N-Cl or N-Mg |
Respective DEN's:
0.0 & 1.8 |
N-Mg is more polar. |
Not really difficult, is
it? Note that, in the last line, N and Cl have about the same EN's.
Only O and F exceed these--but by quite a bit!
